This is what a sufficient scarification by filing looks like on honey locust seeds. I recommend filing deeper than this though, as it makes for slow absorption. I see this depth as a minimum. Filing multiple sides would also speed things up, but isn't necessary.

Note that there are only 2 layers to the seed (from this angle): The seed coat itself (dark brown), and then the cotyledon. Once you see a color change, it means you've gone through the seed coat and that is sufficient to allow water in and hydrate everything.
Using this method, this is what a dry and a saturated seed look like side by side:

Clearly, the seeds do indeed swell significantly, an indication the scarification was successful and the seed is absorbing water. As the seed was swelling, I took measurements of its thickness and plotted it just for curiosity's sake, and here is what that looked like:
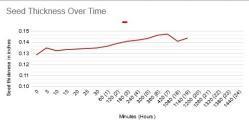
The internet as well as my book said to soak the seeds for 24 hours, which is why the x-axis goes that long. However, by the time the seed had been soaking for ~19 hours, the seed coat was so broken down and soft that my calipers would deform it, and it was no longer possible to get an accurate measurement. However, it's clear that, generally, the scarification worked fine and was allowing the seed to absorb water and break down the seed coat. The blip at the beginning was an error, but I don't know the true value and just had to leave it as is. The large gap in time is just from when I was sleeping.
That's it, easy as that. The seed is ready to sow when it's swollen. As mentioned in the paper, they require no stratification so this swollen seed, assuming it's viable, should germinate in under 4 weeks (
https://www.treeseedonline.com...).
-
This is more of a guide than anything. I wanted to compile this information, hoping that it would be useful to someone in the future.
I got some seeds from a local tree to use as companion species for my tulip poplars (I read a paper that said poplars grown near black locust trees, because of their nitrogen fixation, grew larger diameter trunks). From what I read, honey and black locust fixate nitrogen the same way, so I feel comfortable substituting one species for another in this scenario.
When researching how to treat my seeds, I also saw several studies that showed that acid scarification was the best method and provided the best results.
The link for one such study is here:
https://www.researchgate.net/p...
From the abstract: "It can be concluded that acid scarification without stratification is optimized in breaking honey-locust seed dormancy."
Which is well and good, except I don't have any acid, and could not find anything online about acid-products at home that could be used. So while that is probably the best way to do it, it's not very approachable for the casual honey locust seed grower. Other options were putting them in hot water, or filing them. Filing, I think, is the easiest way for the casual to scarify these seeds. Without being able to find anything online about how far to scarify and what that would look like, I decided to do it myself and measure a seed to make sure my scrape was adequate and allowed the seed to swell (I also saw several sources say that the seeds swelled to 3 times their size as they hydrated, and I was curious to measure this size difference).
Hopefully this type of post is allowed, I searched around a little bit and I felt this was the best place to put it.









