I plumbed only out of necessity, when I studied auto mechanics using anti-seize (an ABSOLUTE must with current alloy content) was hammered into out heads BUT sadly Detroit put cost over reality and there were lot of galvinic problems they knew and ignored.
I much prefer plumbing with automotive fittings than compression fittings, prefer galvinized pipe over plastic.
I do not study the paper labels on hoses , I have faucets that are ferrous and others that are copper alloy (brass) and some plastic crap also.
I have a bottleof anti-seize and use now more often than the tape so many think cures all ills if I am apprehensive on materials used.
Corrosion & Corrosion Resistance
Galvanic Corrosion
The issue of corrosion poses an extreme concern in design. One
of the first questions a designer must address when analyzing a
fastener application is whether the fastener will be subjected to a
corrosive attack during service. It is important to understand that
there are several different types of corrosion including galvanic
corrosion, concentration-cell corrosion, stress corrosion, fretting
corrosion, pitting, and oxidation. The most common form of
corrosion is rust (oxidation) associated with steel structures and
fasteners, although the effects of corrosive attack can be seen in
many other structural materials.
Corrosion can be thought of as an electro-chemical action in which one metal is changed into a chemical
or simply eaten away. When two metals are in contact with
each other in the presence of some electrolyte, the less active
metal will act as the cathode and attract electrons from the
anode. The anode is the material which corrodes.
A simple means of visualizing what is occurring is to consider
the action of a battery. If two metals are immersed in an acid,
a saline, or an alkaline solution, a battery is formed. This
battery produces a flow of electrons between the two metals.
The flow of electrons continues as long as the metals exist, the
solution remains acidic, saline, or alkaline, and as long as a
conductive path connects the two metals.
In the case of galvanic corrosion, the combination of two
dissimilar metals with an electrolyte is all that is needed to form
a reaction. The use of dissimilar metals in structural design is
fairly common, particularly cases where the fastener material is
different from the structure being joined. Furthermore, the
electrolyte may be present in the form of rain, dew, snow, high
humidity, ocean salt spray, or even air pollution. Thus,
designers must take into account the reactivity of the metals
being joined.
All metals have some kind of electrical potential. The
"Galvanic Series of Metals and Alloys" chart above provides a
realistic and practical ranking of metallic electrical potentials.
The alloys near the bottom are cathodic and unreactive; those
at the top are most anodic. The various metals which are
grouped together are reasonably compatible when used
together; those in different groups may cause a corrosion Rev 3-4-2009
problem. Some metals, especially those with significant contents of nickel and chromium, are included in
the table in both their active and passive conditions. Passivation, (i.e.: surface cleaning and sealing)
lowers the metals electrical potential and improves its corrosion behavior. As the series suggests, steel and
aluminum are relatively compatible, but if brass and steel contact, the steel will corrode because it is more
anodic than the brass.
If brass and aluminum plates are connected by a passivated 304 stainless steel bolt, both the brass and the
aluminum will corrode severely where they touch the stainless steel
because they are much more anodic than stainless steel. The
aluminum plate will corrode more heavily due to it being more
anodic to stainless steel than brass is. The aluminum will also
corrode where its exposed surface contacts the brass plate because
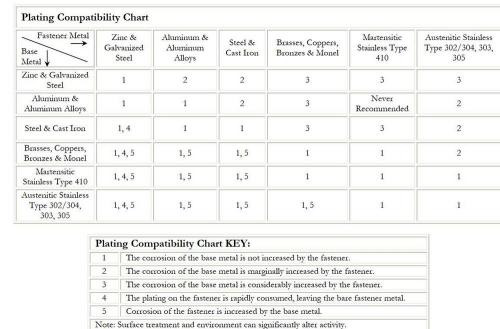
brass is more cathodic. A Plating Compatibility Chart is provided
above that may be used to aid with fastener selection based on
galvanic reaction
